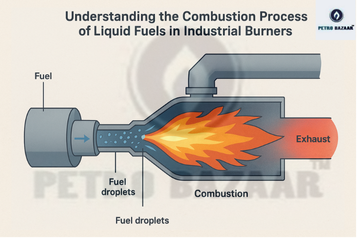
Process of Combustion for Liquid Fuel:
- Pressurisation
- Atomisation
- Vaporisation
- Combustion
Pressurisation: Fuel pressurising means raising fuels pressure (by pump or compressor) to move it through lines, control flow, and prepare it for proper injection or atomisation. In industrial burners, fuels pumps develop enough pressure to overcome piping losses, control valve pressure drops, and burner nozzle requirements while maintaining steady flow.
Atomisation: Fuel atomisation is the mechanical breakup of liquid fuels into fine droplets so that evaporation and mixing with air are fast and uniform.
Common methods include:
- Pressure jet nozzles (using fuels pressure through small orifices),
- Air/steam assist atomisers (using high‑velocity air or steam to shear the liquid),
- Mechanical rotary or spinning cup atomisers (centrifugal breakup). Finer atomisation increases surface area, improves mixing, reduces unburnt fuels, and stabilises the flame, but requires correct pressure, viscosity, and nozzle design.
Vaporisation: Fuel vaporisation is the phase change from liquid droplets to fuels vapour, which is the form that actually burns. After atomisation, droplet heating and evaporation occur in the hot combustion zone, controlled by droplet size, fuel volatility, temperature and relative velocity of hot air or flue gases. Light fuels like petrol and LPG vaporise readily at lower temperatures, while heavier fuels (FO, LSHS) need higher temperatures, preheating, and good atomisation for complete vaporisation before leaving the flame zone
Combustion: Fuel combustion is the chemical reaction of fuels vapour with oxygen, releasing heat, forming products (mainly CO₂ and H₂O for complete combustion) and generating a flame.
Combustion quality depends on:
- Correct fuel–air ratio (stoichiometric or with controlled excess air),
- Adequate turbulence/mixing and residence time,
- Proper ignition energy and stable flame anchoring,
- Sufficient temperature to sustain reaction. Poor atomisation or incomplete vaporisation leads to incomplete combustion, visible smoke, high CO, unburnt hydrocarbons, and deposits, while correct design and control yield high efficiency and lower emissions.